Anestesia


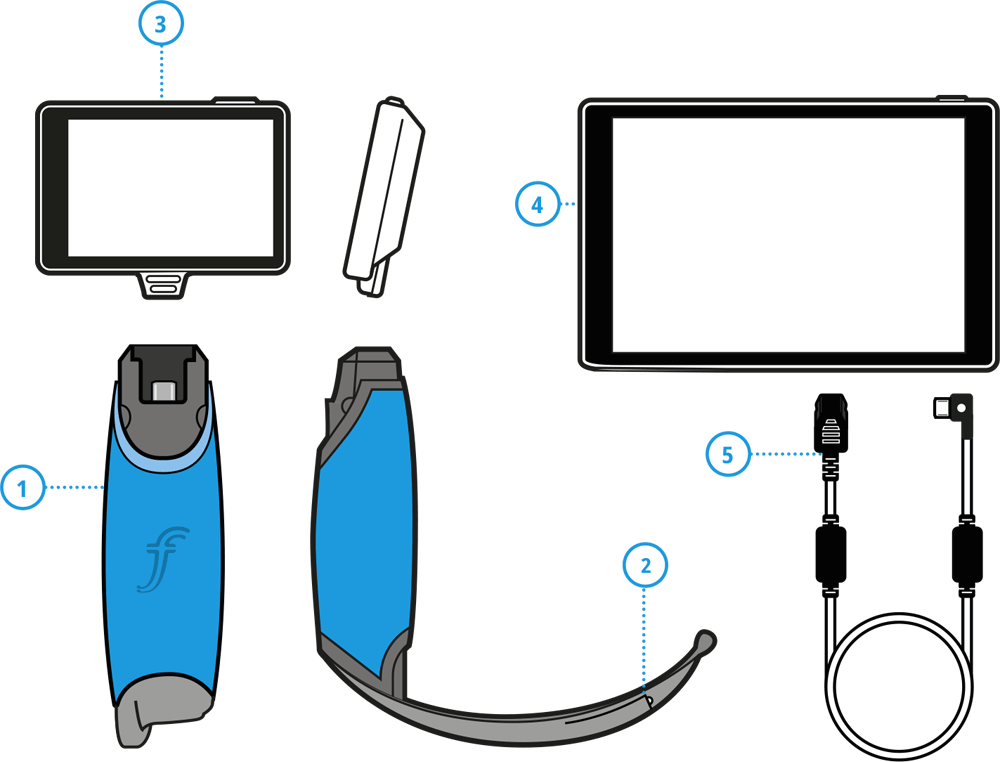

PRODUCT KEY
- Single Use Handle & Blade Assembly
- LED & Camera Assembly
- ProVu 3.5” Display*
- ProVu 8” Display*
- ProVu Amplifier Cable*
Intended Use
The ProVu Video Laryngoscope (ProVu VL) is a sterile, single use portable video-enabled laryngoscope designed for use by qualified medical professionals intended to assist with direct or indirect laryngoscopy in routine or difficult airways. The device is connected to a ProVu Display to provide a video stream from the distal end of the ProVu VL blade. It can be used to facilitate tracheal intubation for patient ventilation for hospital use only and is intended for adult and paediatric patients.
Product Description
The ProVu VL is a portable, rigid, laryngoscope system designed to assist with direct and indirect laryngoscopy in routine and difficult airways by giving a clear view of the patient’s airway using video technology.
The device consists of a single use handle and blade with camera that connects to either a reusable 3.5” Display or reusable 8” Display according to user preference.
Precautions
- Do not use if packaging is open or damaged.
- Do not use after expiry date.
- Always check light operation to ensure sufficient illumination.
- Ensure no lubricant covers the camera lens.
- Ensure the ProVu Display is securely fixed to the ProVu VL handle when in use.
- If the camera view fails, use direct eye view.
- This device is not user serviceable.
- User should familiarize themselves with the camera position in relation to distal tip of blade before use. Failure to do so may lead to harm to the patient.
Warnings & Cautions
- Do not reuse this device. Reuse may result in hazards such as, but not limited to; cross contamination, damage to device, patient injury and / or suboptimal performance.
- No modification of the device is allowed.
- The ProVu VL should only be used by trained personnel.
- Intubation and extubation should be performed using currently accepted medical techniques.
- Apply good medical judgement when using this product.
- Do not point the light guide directly in the eye while in operation. The light output can cause severe eye discomfort.
- Only use the ProVu VL with the ProVu accessories.
- Do not use any part of the system with other laryngoscopes.
- Do not use within Magnetic Resonance Imaging (MR/MRI) environments or with other high interference sources.
- Do not use in procedures which will involve the use of a laser beam or electro-surgical active electrode in the immediate area of the ProVu VL.
- Do not use with a defibrillator.
- Battery is non-replaceable.
- Do not attempt to charge battery whilst in use.
- Do not perform maintenance on device during use.
- Do not use if there are any unintended sharp edges on the product.
- ProVu VL contains a light source that can exceed 41˚C when used at the upper limit of its operating temperature. Patient tissue damage can occur, if in direct contact with this light source.
Any serious incident that occurs in relation to the device should be reported to the manufacturer and the competent authority of the Member State of the user and/or patient.
System Functions Overview
- The ProVu VL features a single-use blade and handle that connects to either the ProVu 3.5” Display, or the ProVu 8” Display.
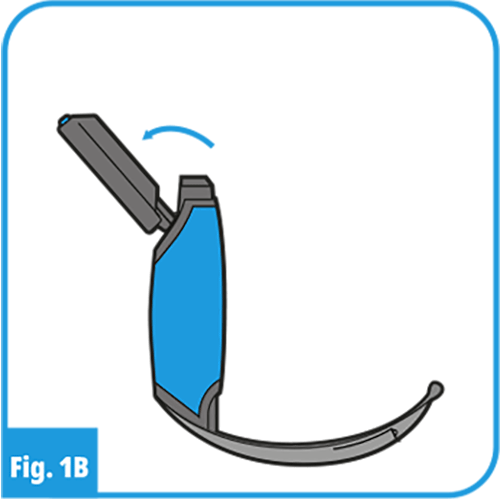
- Once connected the display can be tilted to obtain an optimal view (ProVu 3.5” Display only).
Instructions for Use
- Following the guidelines set out in the ProVu 3.5” Display operating manual (IP0000-384) or ProVu 8” Display operating manual (IP0000-
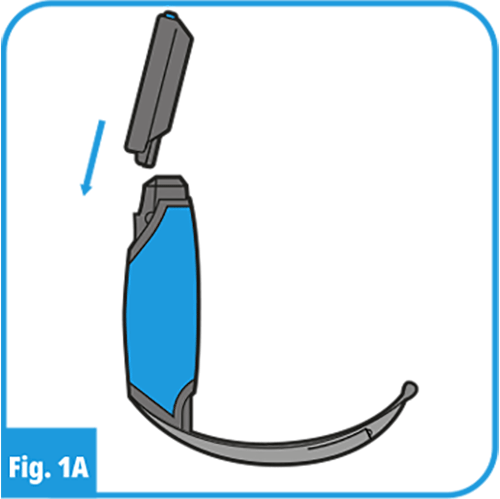
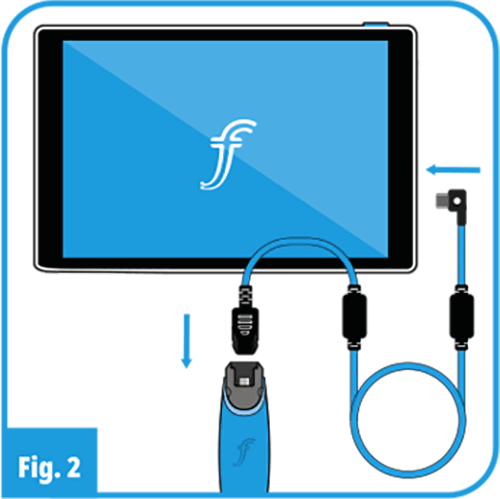
032), ensure that there is enough charge to carry out the procedure and that the Display is ready for use. - Securely connect the ProVu VL Handle and Blade to the ProVu Display selected as shown in Figures 1A and 2.
- Check that the ProVu Display screen shows a live image from the camera with clarity and proper orientation.*
- Use currently accepted medical techniques for intubation, when using the video stream on the ProVu Display as a guide for the intubation.
If space is restricted, the display can be tilted when directly connected to the ProVu Handle and Blade, as shown in Figure 1B. - With the ProVu VL system, extubate the patient using currently accepted medical techniques.
After Use
- After use disconect the ProVu VL Handle and blade from the ProVu Display.
- Dispose of the single-use ProVu VL Handle and blade in accordance with local policies and / or as advised by your local healthcare provider.
- Follow the cleaning guidelines set out in the ProVu 3.5” Display operating manual (IP0000-384) or ProVu 8” Display operating manual
(IP0000-032) for the reusable Display.
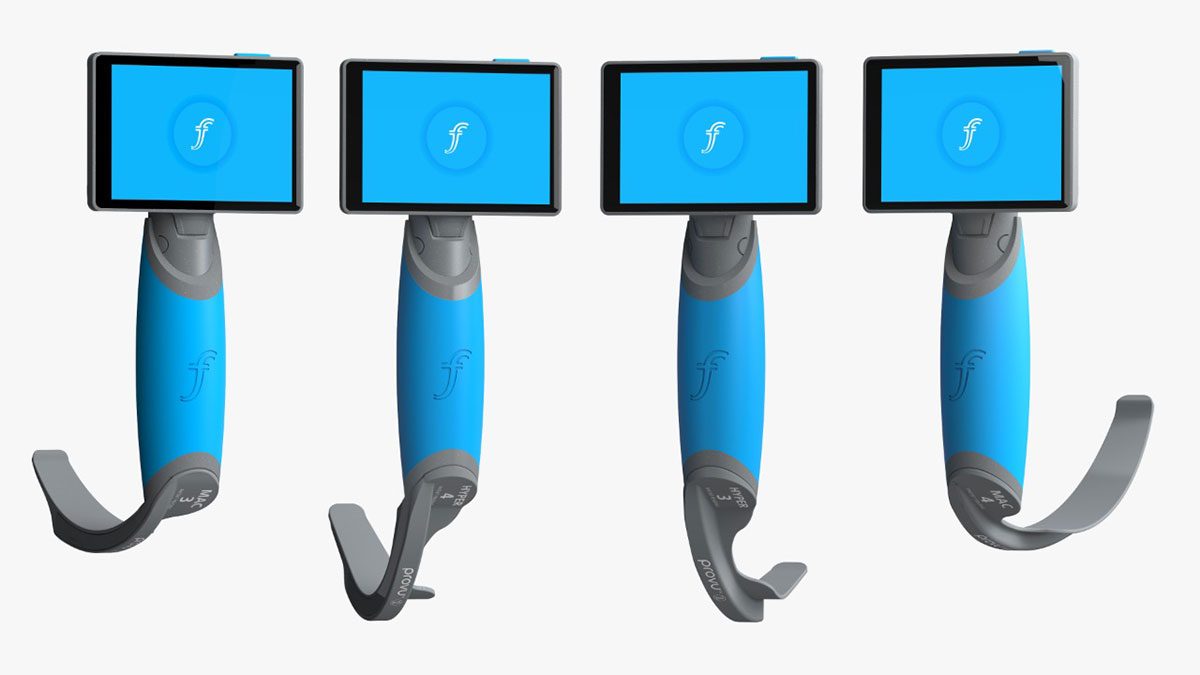
Recommended Size by Patient
- ProVu Single Use Video Laryngoscope Handle with Mac Blade Size 3 (040-08-0130U)
- ProVu Single Use Video Laryngoscope Handle with Mac Blade Size 4 (040-08-0140U)
- ProVu Single Use Video Laryngoscope Handle with Hyperangulated Blade Size 3 (040-08-2130U)
- ProVu Single Use Video Laryngoscope Handle with Hyperangulated Blade Size 4 (040-08-2140U)
List of Accessories
The ProVu VL is designed for use with the following accessories (sold separately)
- ProVu 3.5” Display
- ProVu 8” Display
- ProVu Amplifier Cable
Environmental Conditions
Operating:
10°C to 40°C
≤85% R.H (non-condensing); 70-106kPa
Transport:
-5˚C to 45˚C
≤85% R.H (non-condensing); 70-106kPa
* If no image is seen refer to to ProVu 3.5” Display operating manual (IP0000-384) or ProVu 8” Display operating manual (IP0000-032) troubleshooting
guide where necessary.
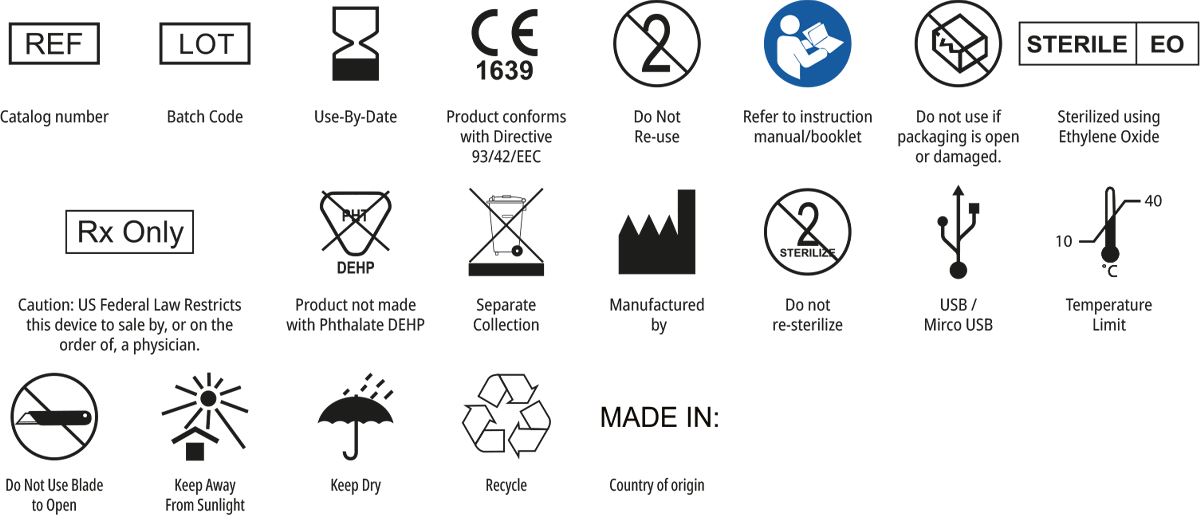
Symbols Glossary